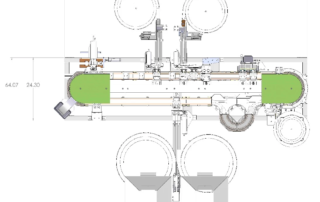
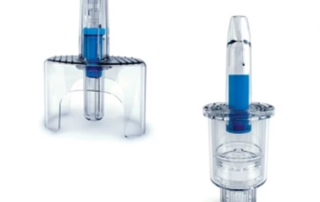
TurboFil offers two distinct systems for the filling and assembly of the Aptar single shot nasal unidose applicators (UDS for disposable use). These applicators allow for a small accurate amount of drug to be administered nasally without the need for an injection or a healthcare professional [1]
A fully automatic system utilizing our Versitrack conveyor platform is ideal for medium scale production. It can handle highly accurate filling, precise placement of the stopper, as well as assembly of the vial, vial holder and actuator. Operation is based on a series of interchangeable holding pucks that are independently controlled, and move the components from station to station for processing.
For products in the development or trial stage, we offer a semi-automatic workstation. This workstation, based on a starwheel approach, fills and places the stopper automatically, while other functions are carried out by operators at the pace of the machine.
The UDS units can be filled with either a peristaltic or piston pump (316L stainless steel, or ceramic), based on product compatibility and customer preference. All product and component contact parts are made of 316L stainless steel, ceramic, or FDA approved plastics.
Because of TurboFil’s deep design expertise, this system can be fully customized to the requirements of your application, including aseptic processing.
[1] According to manufacturer Aptar
Features
-
PLC machine controller (Allen Bradley)
-
Stainless steel vibratory bowl feeders
-
Linear servo indexing conveyor system (Versitrack)
-
Starwheel for indexing (Workstation)
-
Ethernet capabilities for remote diagnostics (option on Workstation model)
-
Liquid filling accuracy +/- 0.5%
-
Suitable for aseptic environments
-
Compact footprint
Speed 80ppm with option of up to 100 ppm adjustable output
Options
-
Dosing system for CIP/ SIP conditions
-
Gas flushing before, during or after the filling process
-
Monitoring and particle counting
-
Automatic rejection of defective devices
-
Process data acquisition software in accordance with FDA 21CFR Part 11
-
RABS (Restricted Access Barrier Systems)
-
IQ/OQ validation package
-
UL508A Certified electrical boxes
-
Advanced data package